-
OMNIgene®·ORAL (OME-505)
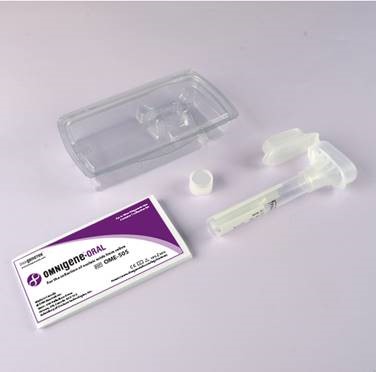
All-in-one system for the self-collection, stabilization, transportation, and storage of microbial nucleic acids from saliva. Stabilizes SARS CoV-2 RNA for up to 21 days i at room temperature. CE/IVD for In Vitro Diagnostic use.
Learn more -
ORAcollect®·RNA (*ORE-100)
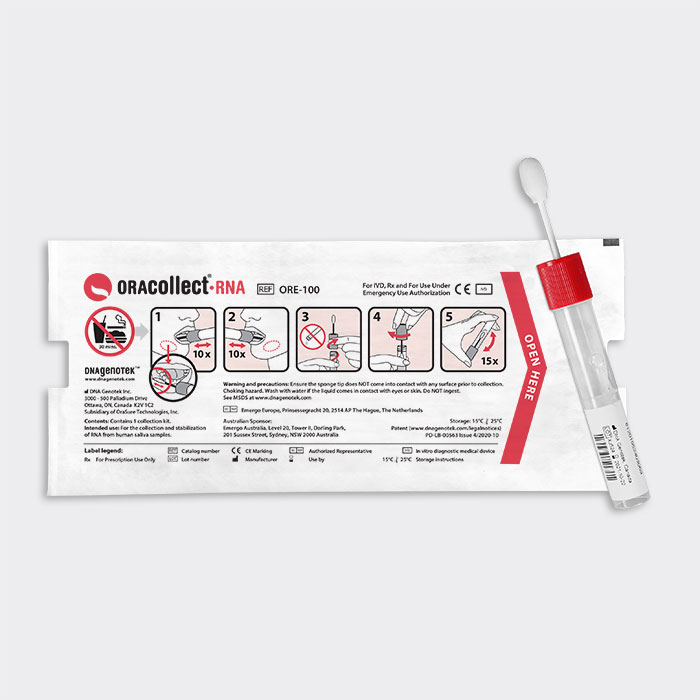
Quick and reliable self- or assisted collection, stabilization and transportation of SARS-CoV-2 from saliva.
Learn more - More than 99% inactivation of SARS-CoV-2 in collected samples ii ,maximizing the safety for healthcare providers, individuals at home, mail personnel during sample shipping, and laboratory staff.
- Collection devices and stabilization chemistries do not contain guanidinium.
- Successfully utilized in qPCR/RT-qPCR assays iii and sequencing-based COVID-19 testing.
- At-home collection without the challenges associated with clinical or hospital settings
- Intuitive design to maximize ease-of-use, optimize donor experience and reduce self-collection errors.
- Bacteriostatic chemistry inhibits the growth of bacteria from time of sample collection to processing.
- Barcoded to improve sample traceability and facilitate the automated sample processing necessary to support large population-based studies
- Maintains sample integrity throughout temperature fluctuations and room temperature storage, eliminating the need for cold-chain transportation or post-collection freezer storage.
- MagMAX™-96 Viral RNA Isolation Kit (Applied Biosystems™, Cat# AM1836)
- MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit (Applied Biosystems™, Cat# A42352)
- Maxwell® HT Viral TNA Kit, Custom (Promega, Cat# AX2340)
- Mag-Bind® Viral DNA/RNA 96 Kit (Omega Bio-tek, Cat# M6246-03)
- Quick-DNA/RNA Viral MagBead (Zymo Research, Cat# R2140 or R2141)
- Collect a saliva sample using an ORAcollect•RNA or OMNIgene•ORAL kit, according to the instructions provided with the kit.
- Incubate the sample at 50°-56°C for 2 hours in a dry incubator (or for 1 hour in a water bath).
- Determine the aliquot size you will extract using your RNA extraction kit and transfer a sample aliquot of that size to a new tube.
- Proceed through your extraction protocol through the addition of the lysis buffer. In some kits, the lysis and binding buffers are combined and may be called binding solution.
- After the lysis buffer has been added, observe the sample for precipitate formation. Precipitate may be observed as the sample turning cloudy. The precipitate usually forms within 60 seconds and is white in colour.
- Prepare two modified lysis buffers as follows:
- a. Lysis buffer with 5% Triton X-100: combine 19 parts lysis buffer with 1 part Triton X-100.
- b. Lysis buffer with 10% Triton X-100: combine 9 parts lysis buffer with 1 part Triton X-100.
- Repeat the compatibility test described above using these modified lysis buffers in place of the unmodified lysis buffer. Precipitation should not occur.
- Policy for Coronavirus Disease-2019
- Templates for EUA submissions for CLIA labs, home specimen collection and commercial manufacturers: In Vitro Diagnostics EUAs
- FDA EUA contact information: CDRH-EUA-Templates@fda.hhs.gov
- If the laboratories are performing testing using EUA-authorized test kits purchased from commercial manufacturers or their distributors.
- If the laboratories are performing testing using test kits purchased from commercial manufacturers or their distributors, where such tests are listed on the FDA notification list.
- If the laboratories are offering at-home testing, including at-home specimen collection. At-home specimen collection devices must be specifically authorized under EUA for the laboratories offering COVID-19 tests.
DNA Genotek products for COVID-19
DNA Genotek’s saliva collection devices offer a robust and safe collection and stabilization approach for non-invasive and self-administered collection and stabilization of saliva for use in validated assays for the detection and identification of the SARS CoV-2 RNA virus (the coronavirus that causes COVID-19). DNA Genotek kits are used in laboratory-based tests for COVID-19 for back-to-work and back-to-school programs, population screening applications, and laboratory testing applications.
The DNA Genotek team has responded rapidly to the spread of COVID-19 by investing in expanded production capacity. The company has scaled up the manufacture of saliva collection devices to meet increased customer demand for COVID-19 testing.
Contact us for detailsKey features and benefit
Featured Content
Literature
RNA Extraction and Downstream Assay Resources
| DNA Genotek Device | Extraction Kit | Manufacturer |
|---|---|---|
| OME-505 | MagMAX 96 Viral RNA (Cat# AM1836) | Thermo Fisher |
| MagMAX Viral/Pathogen (Cat# A42352) | Thermo Fisher | |
| Maxwell HT Viral TNA (Ct# AX2340) | Promega | |
| Mag-Bind Viral DNA/RNA 96 (Cat# M6246-03) | Omega Bio-tek | |
| Quick-DNA/RNA Viral (Cat# R2140 or R2141) | Zymo Research | |
| MinElute Virus Spin | QIAGEN | |
| ORE-100 | MagMAX 96 Viral RNA (Cat# AM1836) | Thermo Fisher |
| MagMAX Viral/Pathogen (Cat# A42352) | Thermo Fisher | |
| Maxwell HT Viral TNA (Ct# AX2340) | Promega | |
| Mag-Bind Viral DNA/RNA 96 (Cat# M6246-03) | Omega Bio-tek | |
| Quick-DNA/RNA Viral (Cat# R2140 or R2141) | Zymo Research |
| DNA Genotek Device | PCR assay | Manufacturer |
|---|---|---|
| OME-505 | TaqPath RT-PCR COVID-19 Kit (Cat# A47814) | Thermo Fisher |
| goTaq (with COVID-19 primers from IDT) | Promega | |
| CRL Rapid Response test | CRL | |
| ORE-100 | TaqPath RT-PCR COVID-19 Kit (Cat# A47814) | Thermo Fisher |
| goTaq (with COVID-19 primers from IDT) | Promega |
Ambry Genetics Laboratory EUA letter EUA Authorization Summary
3B Blackbio Biotech EUA letter
DNA Genotek’s ORAcollect·RNA device EUA: EUA letter EUA Authorization Summary
DNA Genotek’s OMNIgene·ORAL device EUA: EUA letter EUA Authorization Summary
Clinical Reference Laboratories (CRL): EUA letter EUA Authorization Summary
MiraDx: EUA letter EUA Authorization Summary
P23 Labs: EUA letter EUA Authorization Summary
Phosphorus Diagnostics LLC: EUA letter EUA Authorization Summary
Quadrant Biosciences Inc: EUA letter
COMING SOON MK-AN-00043: SARS-CoV-2 sample collection and stabilization with OMNIgene®•ORAL and ORAcollect®•RNA, combined with RNA preparation using prepIT®•Q2A reagents as an alternative workflow.(pdf)
1. Are there any special sample collection requirements when collecting a sample for SARS-CoV-2 testing?
There are no special requirements. Collect a saliva sample using the ORAcollect•RNA (OR/ORE-100) or OMNIgene•ORAL (OME-505) device, according to the instructions provided with the kit. Patients must refrain from eating, drinking, smoking and chewing gum for 30 minutes before collecting a sample.
2. Are there any specific preprocessing steps that are required before sample extraction?
Prior to performing RNA extraction, incubate the samples collected using ORAcollect•RNA or OMNIgene•ORAL, in their original tubes, at 50°C for 1 hour in a water bath or 2 hours in an air incubator. This step is mandatory.
3. What is the purpose of the incubation step?
The incubation step helps reduce the viscosity of the sample and ensures that lysis is complete. Failure to perform this step may result in reduced RNA yield and/or quality. Although the stabilizing liquid contained in the ORAcollect•RNA and OMNIgene•ORAL kits has been shown to inactivate the SARS-CoV-2 virus (see: Inactivation of SARS-CoV-2 in samples collected using Oragene®, ORAcollect® and OMNIgene® products (MK-01430) ), the incubation temperature may be raised to 56°C as an additional precautionary step, if desired. This increased temperature will not impact the RNA quality; however, we do not recommend incubation for longer than 2 hours
4. Which extraction methods/kits are compatible with ORAcollect•RNA or OMNIgene•ORAL samples?
The preservative chemistries found in ORAcollect•RNA and OMNIgene•ORAL collection kits were designed to preserve nucleic acids in the sample by inhibiting RNases and preventing chemical degradation. DNA Genotek has tested a number of commercially available RNA extraction kits and has found that the following are directly compatible with OMNIgene•ORAL or ORAcollect•RNA samples:
5. Can RNA extraction methods/kits other than those listed above be used?
We have observed that the lysis and/or binding buffers contained in some commercially available RNA extraction kits may react with the ORAcollect•RNA or OMNIgene•ORAL stabilization liquid. This reaction is visible as the rapid (usually within 60 seconds, but may take up to five minutes) formation of a white precipitate in the sample when the lysis/binding buffer is added. This precipitate, if carried forward into the eluted RNA, may impact downstream applications such as RT-qPCR.
If using an RNA extraction kit not listed above, check its compatibility with OME-505 and/or OR/ORE-100 samples prior to processing patient samples.
6. What is the procedure for testing RNA extraction method compatibility with ORAcollect•RNA or OMNIgene•ORAL samples?

If no precipitation is observed, ORAcollect•RNA and OMNIgene•ORAL samples should be directly compatible with your desired RNA extraction method.
7. What happens if precipitate is observed after following the compatibility steps above?
If precipitate is observed, your chosen RNA extraction kit is not directly compatible with ORAcollect•RNA or OMNIgene•ORAL samples. However, the following steps may be taken to mitigate this incompatibility:
We recommend using the lysis buffer with the lowest concentration of Triton X-100 that prevents precipitation.
Alternatively, if you are unable to add Triton X-100 directly to the lysis buffer (such as when the buffer is sealed within a closed reagent cartridge), you can add the required amount of Triton X-100 directly to each sample. For example, if your instrument adds 250 µL of lysis buffer to the sample during extraction, you would add 28 µL of Triton X-100 to each sample to achieve the ~10% Triton X-100 concentration.
8. Can DNA Genotek devices be used for at-home collection for COVID-19 tests?
Yes, but only as authorized with respective EUAs issued to commercial test kit manufacturers or test kit providers (including laboratories testing at-home collected saliva samples). Each commercial test kit manufacturer or provider must validate its test for an intended use, e.g., home use or health care professional supervised use.
Note: For your specific case or question, please contact FDA directly via email : CDRH-EUA-Templates@fda.hhs.gov
9. Are the FDA EUA Guidance Documents publicly available?
Yes, guidance is publicly available on the FDA EUA website :
Note: For your specific case or question, please contact FDA directly via email : CDRH-EUA-Templates@fda.hhs.gov
10. Does my CLIA lab need an FDA EUA to sell or offer a COVID-19 test?
NO:
YES:
Note: For your specific case or question, please contact FDA directly via email : CDRH-EUA-Templates@fda.hhs.gov
11. As a developer of a SARS-CoV-2 lab developed test, am I required to validate my assay with samples collected using either ORAcollect•RNA or OMNIgene•ORAL?
Yes, validation is required for all COVID-19 testing in accordance with CLIA and FDA requirements. However, certain studies including pre-collection, shelf life and usability of ORAcollect•RNA or OMNIgene•ORAL for at-home collection may not need to be revalidated.
Note: For your specific case or question, please contact FDA directly via email : CDRH-EUA-Templates@fda.hhs.gov
12. As a SARS-CoV-2 test manufacturer, am I required to validate my assay with samples collected using either ORAcollect•RNA or OMNIgene•ORAL and submit for regulatory approval (e.g., FDA EUA)?
Yes, validation is required for all COVID-19 testing in accordance with CLIA and FDA requirements. However, both ORAcollect•RNA and OMNIgene•ORAL have FDA EUA authorization and may be referenced in your SARS-CoV-2 test submission. Certain studies including pre-collection shelf life and usability of ORAcollect•RNA or OMNIgene•ORAL for at-home collection may not need to be revalidated.
Note: For your specific case or question, please contact FDA directly via email : CDRH-EUA-Templates@fda.hhs.gov
13. What is the sensitivity/specificity of your saliva collection devices?
ORAcollect•RNA and OMNIgene•ORAL are sample collection devices and not diagnostic tests on their own. Therefore, SARS-CoV-2 sensitivity and specificity will depend upon the assay being used to detect the virus.
14. Does the ORAcollect•RNA or OMNIgene•ORAL stabilization chemistry contain guanidinium thiocyanate?
No, there is no guanidinium present in any of our devices. Stabilizing chemistries that contain guanidinium are known to produce harmful cyanide gas when mixed with bleach in laboratory cleaning procedures. More information can be found in the following blog article: Laboratory safety and sample collection devices for SARS-CoV-2
15. Can you use saliva collected in ORAcollect•RNA or OMNIgene•ORAL directly with an assay without an extraction step?
Yes. Our quick-to-assay reagent, prepIT•Q2A, is designed to remove inhibitors found in the stabilization chemistries of ORAcollect•RNA or OMNIgene• ORAL, preparing the sample for the downstream assays. More information is available on the prepIT•Q2A product page.
16. Does your product inactivate the virus?
Yes. We recently conducted a study to demonstrate the ability of the stabilizing solution in the OMNIgene product to inactivate the SARS-CoV-2 virus. We determined that the ORAcollect•RNA and OMNIgene•ORAL stabilizing solution provides > 99% inactivation of the virus. Additionally, there was no observable difference between the virus-free negative controls and the virus-spiked test samples. More information is available on our website: Inactivation of SARS-CoV-2 in samples collected using Oragene®, ORAcollect® and OMNIgene® products (MK-01430)
17. Can DNA Genotek devices be used to stabilize viruses other than SARS-CoV-2?
Yes. We have validated OMNIgene•ORAL (OM-505) for the stabilization of a variety of viruses, including DNA and RNA viruses. Two white papers on this topic are available on our website:
i https://dnagenotek.com/ROW/pdf/PD-BR-00372.pdf
ii https://dnagenotek.com/ROW/pdf/MK-01430.pdf
iii See list of EUAs granted above.
* ORAcollect•RNA (OR-100 and ORE-100) kits will be manufactured with a blue double-ended cap instead of a red double-ended cap. To reflect the cap color change, the device catalog numbers will change from OR-100 and ORE-100 to OR-100.025 and ORE-100.025, respectively. Please see the Product Change Notification here.

