- Volumetric collection from 4 mL-40 mL of urine to ensure accurate sampling
- User preferred method of collection over clinician-collected cervical samples, vaginal swabs and traditional urine cups1,2 no cold-chain shipping required 3,4
- Non-invasive alternative for potential biomarker detection and improved user experience
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- For Research Use Only. Not for diagnostic purposes.
- UCM® - 1:3 Preservative to sample ratio for optimal preservation performance
- UAS™ - 2:5 Preservative to sample ratio for optimal preservation performance
- Human and bacterial DNA stability for 30 days at room temperature (20°C to 26°C)
- Human and bacterial RNA stability for 14 days at room temperature (20°C to 26°C)
- Analyte stability under expected transport conditions (-20°C to +40°C)
- Compatible with common automated liquid-handler platforms, column-based and mag-bead molecular extraction kits
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- Preservative solution capable of stabilizing DNA from bacteria and viruses for research applications, such as Human Papillomavirus (HPV), for 7 days at room temperature1
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- Preservative solution capable of stabilizing DNA from bacteria and viruses for research applications, such as Human Papillomavirus (HPV), for 7 days at room temperature1
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- Preservative solution capable of stabilizing DNA from bacteria and viruses for research applications, such as Human Papillomavirus (HPV), for 7 days at room temperature1
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- Preservative solution capable of stabilizing oncological biomarkers1, such as cfDNA, for 7 days at 20°C – 26°C
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- Preservative solution capable of stabilizing oncological biomarkers1, such as cfDNA, for 7 days at 20°C – 26°C
- For Research Use Only. Not for diagnostic purposes.
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- U.S. FDA Class 1 device
- Volumetric collection of first-void urine
- User preferred, easy self-collection
- Preservative solution capable of stabilizing oncological biomarkers1, such as cfDNA, for 7 days at 20°C – 26°C
- For Research Use Only. Not for diagnostic purposes.
- The research from Hendrickx et al. (2025) shows that the Colli-Pee™ UCM™ FV-5010 device was considered an easy-to-use and well-accepted self-sampling device for cervical cancer screening in a Belgian colposcopy referral population.
- These results highlight the possibility of home-based FVU self-sampling as a liquid biopsy in cervical cancer screening where under screened populations could be approached more easily.
- This study from Bell et al. (2024) provides the first analytical validation of the Allplex™ HPV28 HR Detection assay (Seegene, Cat. No. HP10373Z) for use in FVU samples, offering a reliable, non-invasive and practical alternative to cervical samples for human papillomavirus (HPV) detection.
- Aim to evaluate its genotype-specific performance on FVU samples, following optimization of FVU preanalytics.
- 701 FVU samples collected using a Colli-Pee™ device (20 mL with UCM™ preservative), enriched for HPV-positivity (n = 630).
- This study from Latsuzbaia et al. (2024) shows that Tthe clinical sensitivity of OncoPredict HPV Quantitative Typing assay testing for CIN2+ in urine and vaginal self-samples was similar to cervical samples (ratios of 0.99 [95% CI 0.94–1.05] and 1.00 [95% CI 0.96–1.04], respectively).
- A study from Davies et al. (2024) demonstrate that testing of FVU device-collected urine for HPV was superior to standard-pot-collected urine in colposcopy attendees and has promising sensitivity for CIN2+ detection.
- General population HPV testing of FVU device-collected urine will establish its clinical performance and acceptability as an alternative to routine cervical screening.
- Short-term storage at room temperature: 7 days
- Short- to mid-term storage at 4°C: 14 days
- Mid-term storage at -20°C: 7 to 90 days
- Short-term storage at room temperature: 7 days
- UCM® is 1:3 Preservative to sample ratio for optimal preservation performance
- UASTM is 2:5 Preservative to sample ratio for optimal preservation performance
- easily accessible and available in larger quantities
- non-invasive
- collected easily by an individual at home
- complex, often requires additional processing, interfere with biomarker measurements
- invasive
- pose a risk of infection [2]
Colli-Pee® | FV | UCM | UAS - Series of Products

Powering the volumetric, non-invasive self-collection of urine stabilized at room temperature
Colli-Pee™features:
Know more about this product
Colli-Pee Products
Colli-Pee is available as a neat product and prefilled with a preservative in 4 mL, 10 mL, 20 mL and 40 mL configurations.
Infectious
Diseases
![]()
Human
Papillomavirus
![]()
Chlamydia
trachomatis
![]()
Neisseria
gonorrhoeae
![]()
Mycoplasma
genitalium
![]()
Trichomonas
vaginalis
Oncology
![]()
Liquid
biospsy
![]()
Prostate
cancer
![]()
Cervical
cancer
![]()
Bladder
cancer
Multi-omic
Testing
![]()
Genomics
![]()
Proteomics
![]()
Epigenomics
![]()
Metabolomics
![]()
Transcriptomics
![]()
Microbiomics

Colli-Pee™4 mL
Reference#
N00290
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™4 mL RUO
Reference#
N00290_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes for different research purposes, including infectious and sexually transmitted diseases and oncology, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine

Colli-Pee™10 mL
Reference#
N00309
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™10 mL RUO
Reference#
N00309_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes for different research purposes, including infectious and sexually transmitted diseases and oncology, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine

Colli-Pee™20 mL
Reference#
N00055
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™20 mL RUO
Reference#
N00055_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes for different research purposes, including infectious and sexually transmitted diseases and oncology, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine

Colli-Pee™40 mL
Reference#
N00269
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™40 mL RUO
Reference#
N00269_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes for different research purposes, including infectious and sexually transmitted diseases and oncology, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine
PRESERVATIVE PRODUCTS OPTIONS
Urine stability
Urinary analytes are susceptible to enzymatic and chemical degradation. Additionally, in the time between urine collection and processing, analytes can be degraded. Consequently, methods to stabilize urine samples are necessary. The Colli-Pee® device architecture enables immediate mixing of the sample with a urine preservative, improving stability of the urine specimen.
The combination of the Colli-Pee platform with a preservative for urine stabilization can be game-changing. The use of a preservative enables at-home sample collection in the field of urine-based diagnostic testing. The Colli-Pee device filled with preservative is available in various configurations and volumes.
UCM preservative products
Colli-Pee can be prefilled with UCM®, to allow preservation of DNA in urine. Colli-Pee containing UCM® has been CE-IVD marked, and it is registered in several countries outside Europe.
Optimal preservative to sample ratio:
View the products now
UAS preservative products
To extend the preservation coverage to other analytes (RNA, EV, cfDNA) our newest product Colli-Pee® UAS™ has been developed. Colli Pee UAS™ is available since early 2022 in a Large Volumes format for research use only (RUO).
Optimal preservative to sample ratio:
View the products now
New device
DNA and RNA preservative products
Discover the potential of first-void urine to provide high-quality biomarkers and analytes with a non-invasive and easy-to-access sample type. Collect standardized first-void urine in the unique design of the Colli-Pee™ device, which captures a volumetric sample in an innovative, stabilizing solution capable of preserving both DNA and RNA.
View the products now
DNA and RNA PRESERVATIVE PRODUCTS
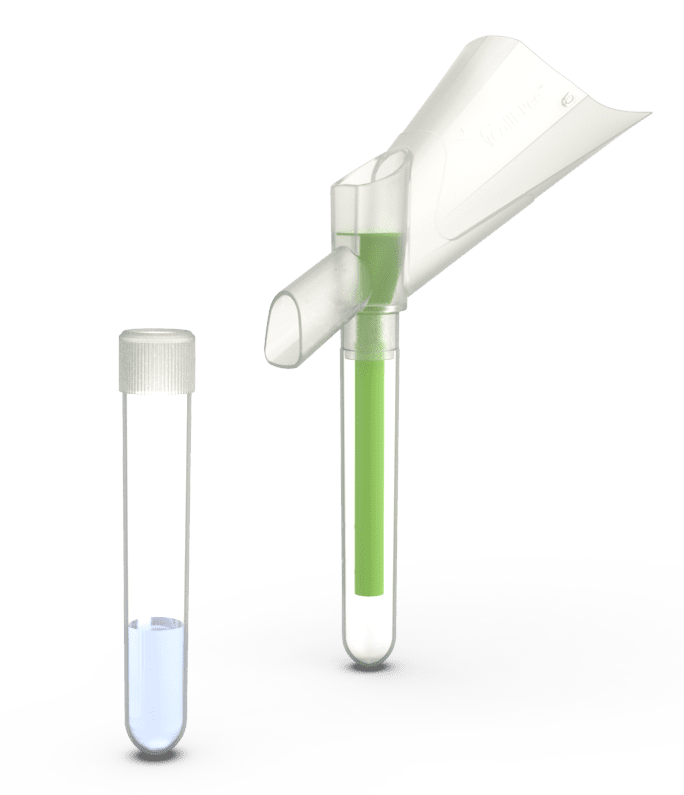
Colli-Pee™DNA and RNA 4 mL
New device
Reference#
N00399_RUO
Collect standardized first-void urine in the unique design of the Colli-Pee™ device, which captures a volumetric sample in an innovative, stabilizing solution capable of preserving both DNA and RNA.
For Research Use Only. Not for diagnostic purposes.

Colli-Pee™UCM 4 mL
Reference#
N00260
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.
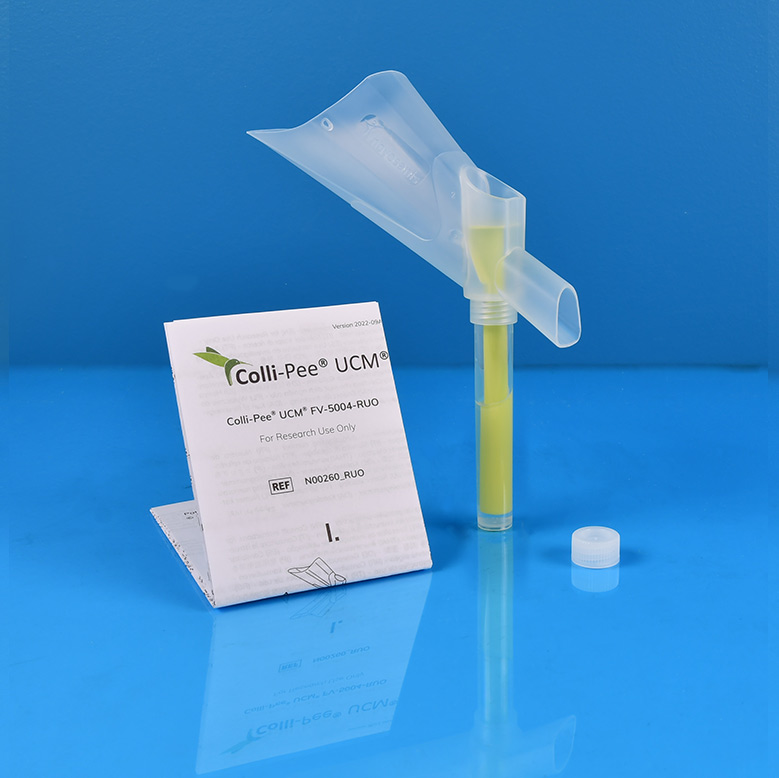
Colli-Pee™UCM 4 mL RUO
Reference#
N00260_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples for the stabilization of DNA from bacteria and viruses1
1 Arora A, Jordaens S, Mehta A, et al. (2020). Novosanis. Why urine preservation is needed for molecular cancer biomarker detection. [White paper].

Colli-Pee™UCM 10 mL
Reference#
N00327
Non-invasive, volumetric, standardized self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™UCM 10 mL RUO
Reference#
N00327_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples for the stabilization of DNA from bacteria and viruses1
1 Arora A, Jordaens S, Mehta A, et al. (2020). Novosanis. Why urine preservation is needed for molecular cancer biomarker detection. [White paper].

Colli-Pee™UCM 20 mL
Reference#
N00176
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™UCM 20 mL RUO
Reference#
N00176_RUO
Non-invasive, volumetric, standardized self-collection of first-void urine samples for the stabilization of DNA from bacteria and viruses1
1 Arora A, Jordaens S, Mehta A, et al. (2020). Novosanis. Why urine preservation is needed for molecular cancer biomarker detection. [White paper].

Colli-Pee™UAS 10 mL RUO
Reference#
N00345_RUO
Preserve oncologic biomarkers such as cfDNA, EV and EV RNA in first-void urine with the Colli-Pee™ UAS™ device. Providing volumetric self-sampling of first-void urine through an easy and user preferred collection method, the Colli-Pee™ UAS™ device is powering non-invasive self-sampling solutions for oncology research applications with first-void urine.
1 Jordaens S, Arora A, MacDonald K, et al. (2023). UAS™-A Urine Preservative for Oncology Applications. Cancers (Basel). Jun 8;15(12):3119. doi: 10.3390/cancers15123119.

Colli-Pee™UAS 20 mL RUO
Reference#
N00339_RUO
Preserve oncologic biomarkers such as cfDNA, EV and EV RNA in first-void urine with the Colli-Pee™ UAS™ device. Providing volumetric self-sampling of first-void urine through an easy and user preferred collection method, the Colli-Pee™ UAS™ device is powering non-invasive self-sampling solutions for oncology research applications with first-void urine.
1 Jordaens S, Arora A, MacDonald K, et al. (2023). UAS™-A Urine Preservative for Oncology Applications. Cancers (Basel). Jun 8;15(12):3119. doi: 10.3390/cancers15123119.

Colli-Pee™UAS 40 mL
Reference#
N00342
Non-invasive, volumetric, standardized, self-collection of first-void urine samples. Offering various sizes to capture a range of first-void urine volumes, the Colli-Pee device is powering non-invasive self-sampling solutions with first-void urine.
Device / Assay manufacturers must validate the use of this urine collection device for use with their device.

Colli-Pee™UAS 40 mL RUO
Reference#
N00342_RUO
Preserve oncologic biomarkers such as cfDNA, EV and EV RNA in first-void urine with the Colli-Pee™ UAS™ device. Providing volumetric self-sampling of first-void urine through an easy and user preferred collection method, the Colli-Pee™ UAS™ device is powering non-invasive self-sampling solutions for oncology research applications with first-void urine.
1 Jordaens S, Arora A, MacDonald K, et al. (2023). UAS™-A Urine Preservative for Oncology Applications. Cancers (Basel). Jun 8;15(12):3119. doi: 10.3390/cancers15123119.
Literature
Clinical accuracy of OncoPredict HPV Quantitative Typing (QT) assay on self-samples
These are compiled answers to the most common questions. If you do not see the answer to your question, feel free to reach out to us.
What is first-void urine?
First void (or first catch or first pass) urine, typically referred to the first 20 mL of urine flow, is collected at any time of the day. This fraction has shown to contain higher concentrations of Human Papillomavirus (HPV) and sexually transmitted infections (STI)-related DNA. First-void urine is also a promising source to detect biomarkers for prostate cancer. For more information visit first-void urine section.
Are all urine fractions the same?
No, not all urine fractions are the same. There is a range of terminology used in the field to characterize the different times and fractions of urine collection. Read our Pee-Glossary to know more.
How can the Colli-Pee be used?
To know how Colli-Pee works, watch our instruction video or read our instructions for use.
Why not simply use a urine cup for the collection of a urine sample?
Collecting a first-void urine sample (first 20 mL of urine) with a classic urine cup can be awkward, messy and inconvenient for the user. The volume of the collected sample is also difficult to control, leading to undesired variation in downstream analysis. The unique design of Colli-Pee® allows for standardized and volumetric collection of first-void urine, without the need to interrupt urine flow.
At what time of the day can the Colli-Pee be used?
Anytime. There is no difference between first-void urine collected with Colli-Pee® in the morning or during the day.
Can Colli-Pee be used with different toilet types or urinal designs?
Colli-Pee® can be used in 3 different pee positions, and is therefore suitable for use with different toilet types or urinals.
Can I use Colli-Pee at home?
Yes. One of the main advantages of Colli-Pee® is that it is very well suited for urine collection at home and for postal delivery, offering the opportunity to reach a wider population. For more information , visit our 'discover more about Colli-Pee in a box for home-based colletion kits' section on this webpage.
Which Colli-Pee variants are currently available?
The Colli-Pee® device is registered in several countries. Availability of the variants depends on your location.
Contact us to know the registration status in your region.
What is the shelf life of my Colli-Pee device?
All neat variants of Colli-Pee® have a shelf life of 5 years. Colli-Pee® Small Volumes with UCM and Colli-Pee® 20 mL with UCM have a shelf life of 2 years.
What is the price of a Colli-Pee collection device?
For more information on Colli-Pee® prices, contact your DNA Genotek sales responsible, or contact DNA Genotek directly.
What regulatory information is available for Colli-Pee?
Our quality system is fully compliant with the requirements of ISO13485 quality standards for manufacturers of medical devices. Colli-Pee® device is CE-marked and FDA listed, with registered trademarks® in several countries. Contact us to know the registration status in your region.
How should I store and transport my urine sample, collected with Colli-Pee?
In the period between collection and processing, analytes in urine can be degraded. We developed Urine Conservation Medium (UCM®), a non-toxic urine stabilization chemistry that will keep the DNA in your urine sample stable at room temperature, for a period of at least 7 days. Furthermore, to extend the preservation coverage to other analytes (RNA, EV, cfDNA) a new preservative, UAS is currently in development.
Which prefilled urine stabilization chemistries are available in Colli-Pee and in which applications can they be used?
Colli-Pee® can be prefilled with UCM, to allow preservation of DNA in urine.
To extend the preservation coverage to other analytes (RNA, EV, cfDNA) we introduce Colli-Pee® containing UAS TM.
How long can I keep my urine sample stable in the presence of a stabilization chemistry?
Storage conditions for Colli-Pee® (20 mL) tubes containing UCM®, after first-void urine collection varies:
Storage conditions for Colli-Pee® tubes containing UASTM, after first-void urine collection varies:
What is the chemistry to urine ratio?
Optimal preservative to sample ratio depends on the type of preservative.
Our data shows:
In which applications can the Colli-Pee be used?
Colli-Pee® can be used in various applications, including detection of sexually transmitted infections (STIs) as well as cancer biomarkers
Which urinary analytes can be detected in urine?
As urine collection is absolutely non-invasive it is very suitable for multi-omic testing, including genomics, epigenomics, transcriptomics, proteomics, metabolomics, microbiomics. Several analytes can be found in urine such as DNA, RNA, proteins, metabolites.
Can Colli-Pee be used for STI detection?
Several studies have shown first-void urine collected with Colli-Pee® with UCM can improve detection of sexually transmitted infections (STIs), including Human Papillomavirus (HPV).
Can urine collected with Colli-Pee be used for cervical cancer screening?
When appropriately sampled, stored, and processed, first-void urine is a particularly valuable liquid biopsy source for primary Human Papillomavirus (HPV) testing. Colli-Pee® has been used previously in various studies around cervical cancer screening, and has been shown to be an optimal sample collection device in this setting.
Why use urine over physician taken cervical/vaginal samples?
Several studies have shown that women prefer easy, and non-invasive techniques that are not clinician dependent. Urine as a sample type is well-accepted, and given its ability to detect Human Papillomavirus (HPV), has the potential to reach a wider population, especially women who do not participate in screening.
Can HPV be detected in male urine samples?
Although Colli-Pee® is perfectly suited to be used by all genders, no publications are available to date. Please contact us if you would like to set up a trial.
Can Colli-Pee be used for prostate cancer detection?
Yes. Colli-Pee® can facilitate and standardize detection and stabilization of urinary biomarkers in prostate cancer research. Colli-Pee® is already used by Dx companies.
Why use urine over blood as a liquid biopsy?
Urine sampling is absolutely non-invasive and allows for home self-sampling. Additionally, collection is not limited by health status of patient, poses no risk for bloodborne pathogen transmission, and allows high volume allowing multi-omic testing.
Can Colli-Pee be used for the detection of cell-free DNA (cfDNA)?
Yes. cfDNA can be detected in various biological fluids, including cerebrospinal fluid, plasma, saliva, seminal plasma, serum and urine.
Can Colli-Pee be used for extracellular vesicle (EV) detection?
Yes, urinary extracellular vesicles (UEVs) entering from the kidneys and urinary tract can be detected in urine collected with Colli-Pee®.
Can urine collected with Colli-Pee be used for methylation tests?
When appropriately sampled, stored, and processed, first-void urine is a particularly valuable liquid biopsy source for primary Human Papillomavirus (HPV) testing and for analysis of molecular biomarkers including methylation.
Colli-Pee and first-void urine
Urine sampling offers several benefits, however, not all urine fractions are the same. First-void urine is considered the first 20 mL to 30 mL of urine flow.
Collecting an accurate first-void urine sample with a standard urine cup can be awkward, messy, and inconvenient for the user. For this reason, we developed Colli-Pee.

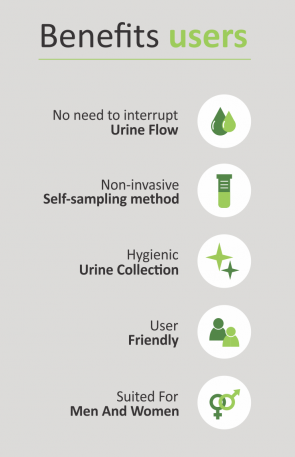
Urine sampling advantages
Urine sampling has multiple benefits:
Blood sampling has several limitations:
First-void urine, also known as first-catch or first-pass urine is collected at any time of the day and is typically referred to the first 20 mL to 30 mL of urine flush. A first-void urine specimen has shown to contain higher concentrations of sexually transmitted infections (STI)- related DNA than other fractions (3,4). Additionally, first-void urine can be important to identify cancer biomarkers (2).
First-void urine contains more DNA and RNA, as well as other analytes, than other fractions such as a random or midstream urine sample (5).
For urological applications, a urine specimen is in many situations the preferred liquid biopsy source.
Given the importance of capturing first-void urine for improved accuracy and test results, we developed Colli-Pee, a first-void urine collection device. The unique design of the device allows easy collection of first-void urine and improved sample preservation, which can be challenging and difficult with a regular urine cup.
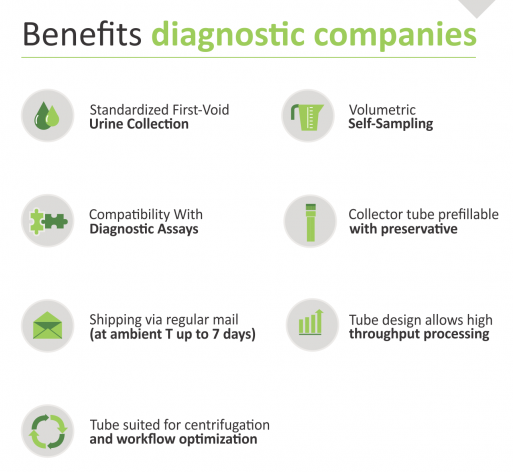
| Colli-Pee™ device provides non-invasive, self-collected and volumetric sampling of first-void urine. | |
| Suited for male and female first-void urine collection in the clinic or at-home without the need to interrupt urine flow |  |
| Comfortable, easy, and more accurate first-void urine collection compared to a regular urine cup |  |
| Available in 4 mL, 10 mL, 20 mL, and 40 mL sample volumes with or without preservative solutions |  |
How does Colli-Pee work?
Visual representation of Colli-Pee components
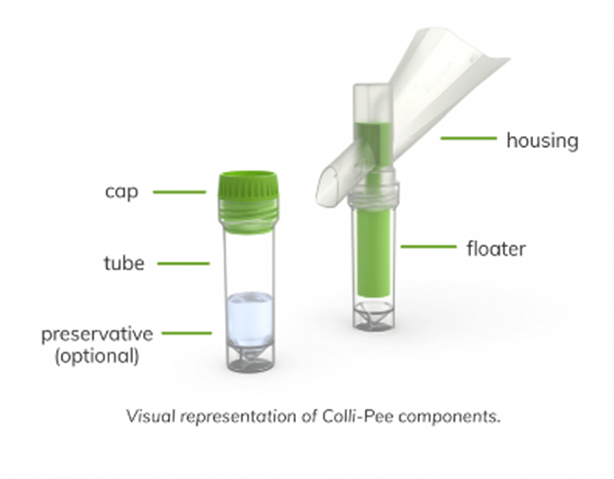
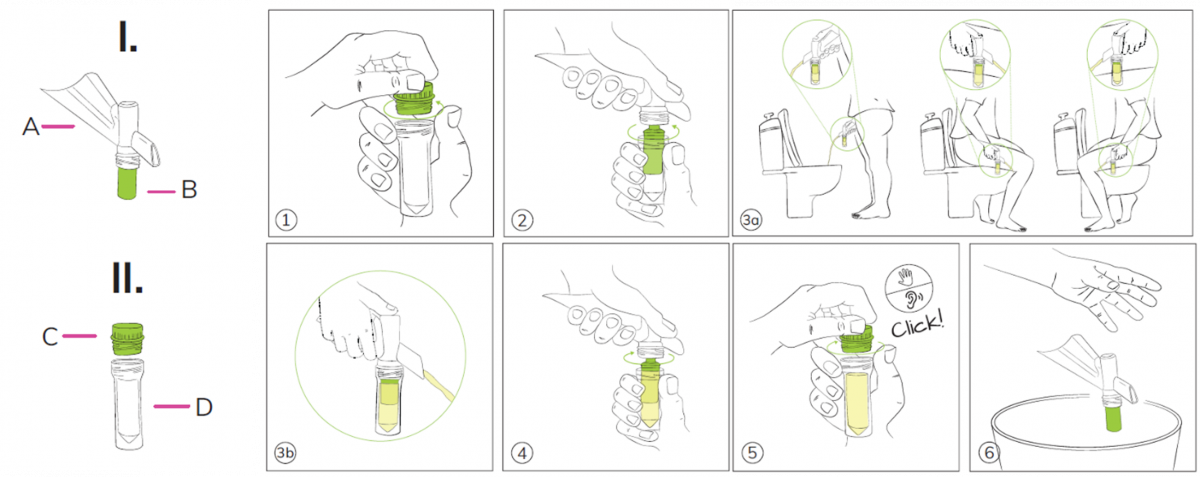
1. Unscrew the tube cap (C).
2. Screw or push the collector tube (D) on the Colli-Pee housing.
3. Collect urine: The device will automatically collect the correct volume
4. There is no need to interrupt the urine stream. Excess urine that no longer fits into the collector tube will flow into the toilet via the other end of the housing
5. Disconnect the collector tube from the Colli-Pee housing.
6. Close the tube tightly. Tube cap (C) should be screwed on tightly.
7. Dispose the Colli-Pee device according to local regulations for plastic waste.
8. Wash your hands.
Inside DNA Genotek
Legal

