- High quality, high quantity DNA †
- Non-invasive, reliable self-collection or assisted collection of samples
- DNA from saliva is a proven alternative to DNA from blood for downstream applications
- Increase donor compliance by 200% over blood collections
- Oragene•Dx nucleic acid stabilization chemistries are bacteriostatic, preventing human DNA degradation and inactivating SARS-CoV-2
- Samples remain stable at room temperature for one year, reducing storage and transport costs
- Compact form factor and robust sample stability across temperatures ranging from -20°C to 50°C increases sample transport options, including postal service
- Increase workflow efficiency and minimize sample handling with a standardized tube format and integrated 1D barcode
- Branding and customizable packaging available to create a unique end-customer experience
- 96.5% of donors agreed or strongly agreed that the instructions were easy to understand
- 96% of donors understood how to collect their saliva sample properly
- 99.5% of donors understood how to send the sample back to the laboratory
- 100% of donors understood where and how to contact technical support for any questions
- 95% of samples passed sample weight criteria (between 1-4 mL of total sample volume)
- 97% of samples had a DNA concentration of 5 ng/μL or more
- 96% of samples passed DNA sequencing call rate criteria
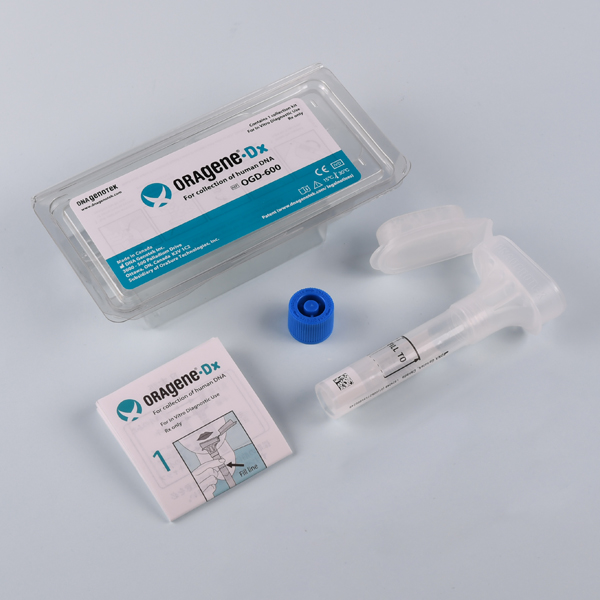
 | OGD-600
| OGD-600
Oragene™ saliva kits for high quality DNA saliva samples for genetic diagnostics
Achieving sensitivity and specificity of a diagnostic test requires a high quality DNA sample. While traditionally blood was considered the preferred sample type for in vitro diagnostics, using saliva samples as a non-invasive source of genomic DNA reduces patient risk and offers convenience with home DNA collection kits.
Oragene™•Dx DNA sample collection kits are a proven viable and beneficial alternative to blood samples, offering a combined saliva collection and DNA stabilization device, that enables efficient storage and transportation of high-quality biological samples at ambient temperature.
Oragene•Dx saliva collection kits are FDA cleared devices†. Leveraging the 510(k) clearance allows molecular test manufacturers to immediately use these saliva collection devices in either a prescription or direct-to-consumer model. Saliva collection can be done by either a healthcare professional at point-of-care or using at home DNA collection kits.
General clearance also offers more customer-friendly customization options, for test manufacturers seeking FDA clearance, this will save both time and money compared to re-validating other devices that are not FDA cleared for general use. Oragene•Dx saliva collection devices can help launch or optimize a more flexible and streamlined direct-to-consumer genetic diagnostics service.
Overview
Oragene™•Dx Saliva Collection Kit Benefits
Oragene•Dx saliva kits collect superior samples and improve patient care by providing a simple, painless alternative while removing the inconvenience, anxiety and cost of going to a clinic for a blood draw. Saliva sample collection also improves compliance rates, speeds up collection and extraction processes, and offers more options for transport and storage when compared to blood, rendering healthcare and genomic diagnostics more efficient.
Oragene™•Dx Saliva Collection Kit Proven Performance
An Oragene•Dx DTC User Comprehension Study* was conducted to assess the acceptability of saliva samples collected with Oragene•Dx devices for a typical direct-to-consumer application. This study involved 212 naïve, adult, general population donors, who received a typical DTC Oragene•Dx saliva collection kit including instructions and two-way mailing box for return shipping
As a result, data obtained from this study demonstrated that participants comprehended the instructions for use provided with the Oragene•Dx device and could successfully provide a saliva sample at home with acceptable quality for use in genotyping.
Proven easy to use*
Proven technical performance*
Oragene•Dx product features
|
|
Oragene®•Dx
|
|---|---|
|
Completely non-invasive
|
✔
|
|
Standardized tube format for automated processing equipment
|
✔
|
|
Sample stability at room temperature
|
1 year
|
|
Bacteriostatic solution
|
✔
|
|
Compact format for mailing
|
✔
|
View donor user instructions
Performance characteristics**
|
OGD-600
|
Yield (µg)
|
Concentration (ng/µl)
|
A260/280 ratio
|
|---|---|---|---|
|
Mean ± SD
|
58.5 ± 47.0
|
68.1 ± 55.3
|
1.7 ± 0.1
|
|
Median
|
48.4
|
55.3
|
1.7
|
|
95% of samples
|
≥ 13.1
|
≥ 16.0
|
1.5 – 1.9
|
For in vitro diagnostic use. For collection of human DNA. Oragene•Dx has been cleared for in vitro diagnostic use in the U.S.A.
† FDA cleared for in vitro diagnostic use for direct-to-consumer applications, K192920. NOTE: Device / Assay manufacturers must validate the use of OrageneTM•Dx for use with their device.
* Based upon results derived from The Oragene®•Dx DTC User Comprehension Study, K192920.
The Oragene®•Dx DTC User Comprehension Study, K192920 was conducted to support a 510(k) submission to FDA. The study was conducted to assess the acceptability of saliva samples collected with the Oragene®•Dx device for a typical direct-to-consumer application. The study demonstrated that customers comprehended the instructions for use provided with the Oragene•Dx device and could successfully provide a saliva sample at home with acceptable quality for use in genotyping.
Full terms and conditions for all DNA Genotek products are available here.
DNA Genotek's sample collection devices and nucleic acid stabilization chemistries are protected by issued and pending patents in numerous countries around the world.
** Data obtained by testing 450 samples from 245 individuals
Literature
Data Sheets
Protocols
Laboratory protocols for manual purification of DNA using DNA Genotek's prepIT•L2P DNA extraction kit
(available in multiple languages)
Videos
Processing of DNA Genotek saliva tubes on SARSTEDT Lab Automation (copyright SARSTEDT AG & Co. KG).
Scientific Posters
User Instructions
Safety Data Sheets
Accessories
 The collection protocol is very convenient, the extraction is very quick and simple, and the yield is great. We have collected from [donors] not willing
to go for a blood draw, [including] children, elderly patients and overseas relatives. The ease of shipping and self-collection are remarkable.
The collection protocol is very convenient, the extraction is very quick and simple, and the yield is great. We have collected from [donors] not willing
to go for a blood draw, [including] children, elderly patients and overseas relatives. The ease of shipping and self-collection are remarkable.
Ruth McPherson, MD, PhD and Nihan Kavaslar, PhD
University of Ottawa Heart Institute
Overcoming challenges in DNA sample collection.
Download this research report to help you increase your compliance rate.
Inside DNA Genotek
Legal