- Standardize your workflow
- Simplify your assay development
- Accelerate your time to market
- Sample stability (30 days at room temperature)
- Sample neutrality (Unbiased representation of the in vivo state)
- Reproducibility (Lot-to-lot and aliquot-to-aliquot)
- Usability (Instructions and execution)
- Compatibility with all sample types (Adult and pediatric cohort)
OMNIgene•GUT Dx | OMD-200
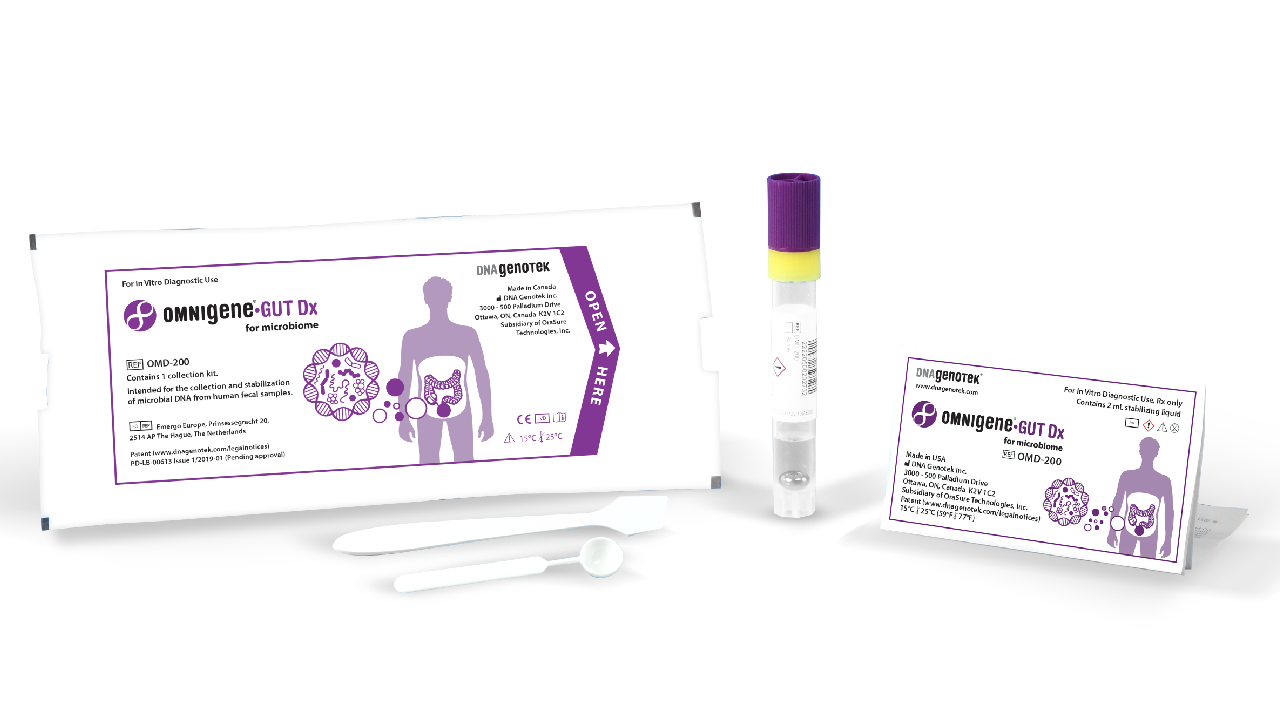
OMNIgene®•GUT Dx is the first and only FDA-authorized collection device (via De Novo pathway) validated for the self-collection and stabilization of bacterial DNA for gut microbiome profiling.
Bringing confidence and accuracy to your microbiome discovery
The FDA granted the OMNIgene•GUT Dx a De Novo authorization based a robust validation of:
View donor user instructions
Literature
Data Sheets
Protocols
Safety Data Sheets
Beyond GUT microbiome
The OMNIgene® line of products also includes all-in-one systems for quantitative profile analysis of the oral and vaginal microbiome.
View All Microbiome Collection Devices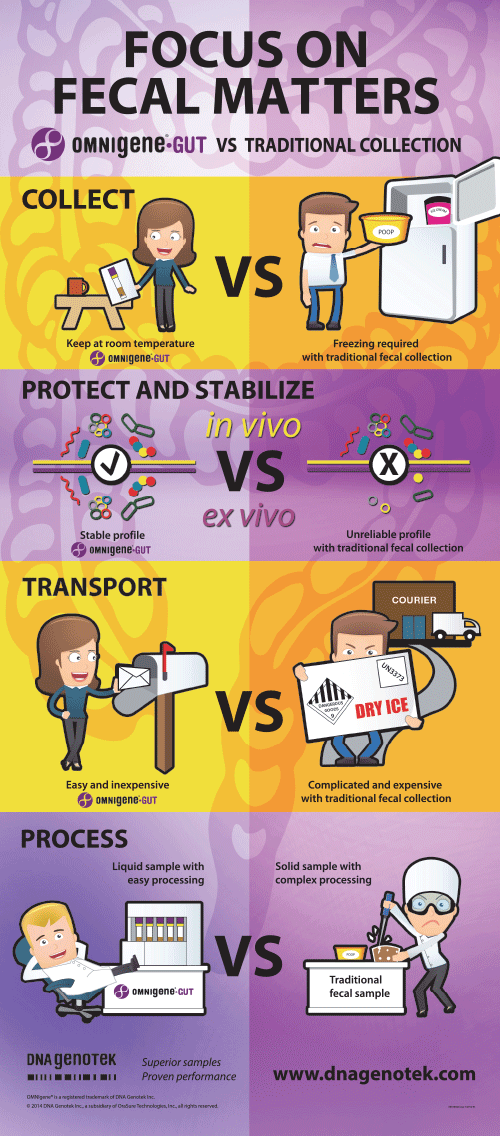
Focus on fecal matters
OMNIgene•GUT vs. traditional collection
Overcoming challenges in DNA sample collection.
Transport and store stabilized DNA at ambient temperature – no cold chain required.
Inside DNA Genotek
Legal