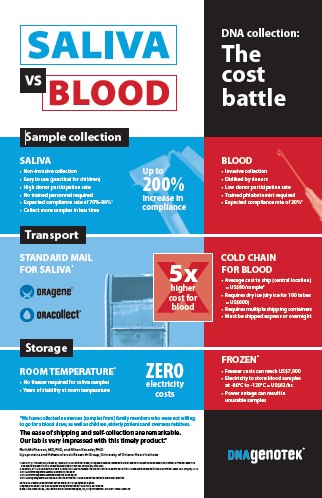
- Non-invasive, reliable collection that increases compliance and decreases costs
- Eliminate phlebotomy costs and requirement for a clinic/hospital setting
- DNA from saliva is equivalent to DNA from blood for downstream applications
- Ideal for use with children or donors that are not able to spit
- Sample remains stable for years at room temperature, reducing transportation and storage costs
- Sample can be mailed using the standard postal system
- Increase efficiency, minimize sample handling and reduce handling errors with a compatible format for high-throughput processing
 |
OGR-575
|
OGR-575
For assisted collection
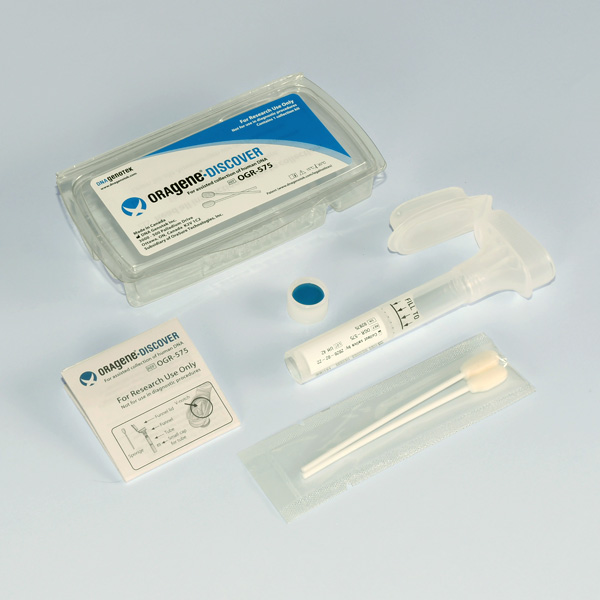
Collect superior samples for your genetic analysis
Are you looking for a non-invasive way to collect DNA from patients that cannot or choose not to comply with blood collections? DNA Genotek has developed a product for collecting high quantity and high quality DNA from saliva using absorbent sponges. This method is an all-in-one system for the collection, stabilization and transportation of DNA from saliva.
Overview
Product Benefits
View donor user instructions
Collection method comparison chart
Blood Collection |
Oral Collection |
|||
Attributes |
Venous blood |
Mouthwash |
Buccal swabs |
Oragene•DISCOVER (OGR-575) |
|---|---|---|---|---|
Non-invasive collection |
✘ |
✘ |
✔ |
✔ |
Specimen stability at room temperature |
Days |
Weeks |
Days |
Years |
Low bacterial content |
✔ |
✘† (up to 60% bacterial content) |
✘† (up to 90% bacterial content) |
✔ (median 6.8% bacterial content) |
Median DNA yield |
30 µg |
35 µg |
2 µg |
17.3 µg‡ |
Sample size |
1 mL |
10 mL** |
1 swab |
0.75 mL |
Molecular weight |
> 23 kb |
> 23 kb |
< 23 kb |
> 23 kb‡ |
Shipping at ambient temperature |
✘ |
✔ |
✔ |
✔ |
Ideal for non-compliant donors |
✘ |
✘ |
✘ |
✔ |
† Birnboim, H.C., Iwasiow, R.M. and James, C.M.P. (2008). Human genomic DNA content of saliva samples collected with the Oragene self-collection kit. DNA Genotek. PD-WP-011.
‡ Niles, J.O., Rabuka, S., and Iwasiow, R.M. (2010). Non-invasive, assisted collection of high quantity and quality genomic DNA from saliva of young children. DNA Genotek. PD-WP-018.
** Volume of mouthwash used for sample collection.
Oragene•DISCOVER is for research use only, not for use in diagnostic procedures.
Full terms and conditions for all DNA Genotek products are available here.
DNA Genotek's sample collection devices and nucleic acid stabilization chemistries are protected by issued and pending patents in numerous countries around the world.
Literature
Data Sheets
Safety Data Sheets
Collect & Transport
Collect & Transport
Oragene collection instructions
Instructions for self or assisted collection in multiple languages.
Mailing accessories and shipping recommendations
DNA Genotek has created a mailing solution that complies with International Air Transport Association (IATA) regulations for samples not expected to be pathogenic, while also protecting the collection kit on the way to and from the donor.
Customization options
Storage & Stability
Videos
Yield & Quality
Yield & Quality
PD-WP-00031: Impact of population and laboratory methods on DNA yield and variability
Summary of the total DNA yield from Oragene/saliva samples as reported in peer-reviewed publications.
PD-WP-011: Human genomic vs bacterial DNA content of Oragene/saliva samples
Quantitative information about the amount of human genomic and bacterial DNA in Oragene/saliva samples, as estimated by two different methods.
MK-AN-017: Tips to improve the A260/A280 ratio of Oragene-purified DNA samples
Despite the fact that sample turbidity (i.e., absorbance at A320) does not affect down-stream analysis, some users have enquired about modifications to our standard protocol that will improve this ratio. In this document, we describe 3 alternative methods that can be used to obtain high A260/A280 ratios from all samples.
Lab Protocols
Lab Protocols
Protocols
Manual Purification
Compatibility with Automated Purification
Quantification
MK-AN-017: From turbidity to clarity: Simple methods to improve the A260/A280 ratio of Oragene-purified DNA samples
Despite the fact that sample turbidity (i.e., absorbance at A320) does not affect down-stream analysis, some users have enquired about modifications to our standard protocol that will improve this ratio. In this document, we describe 3 alternative methods that can be used to obtain high A260/A280 ratios from all samples.
PD-PR-040: RNA removal within Oragene/saliva samples
This protocol describes the use of double-RNase digestion to remove the RNA in Oragene/saliva samples.
PD-PR-065: Bacterial DNA assay
The purpose of this assay is to determine the bacterial DNA content of purified DNA from Oragene/saliva samples.
Downstream Applications
Downstream Applications
Accessories
 This is a truly great product. I have tried many different chemistries to obtain DNA… and I have not seen a better compromise for yield, purity, time to extract and ease of collection than Oragene.
This is a truly great product. I have tried many different chemistries to obtain DNA… and I have not seen a better compromise for yield, purity, time to extract and ease of collection than Oragene.
Mehdi Keddache, Coordinator
Cincinnati Children's Hospital, DNA Sequencing Services
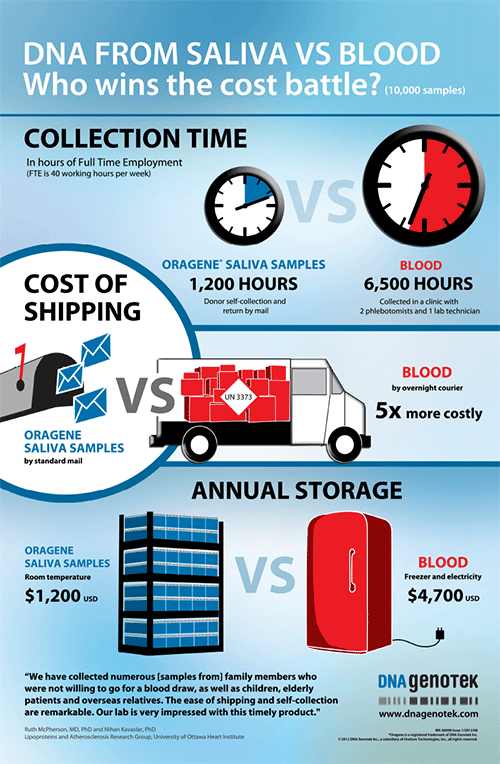
DNA from saliva vs. blood
Who wins the cost battle?
Overcoming challenges in DNA sample collection.
Download this research report to help you increase your compliance rate.
Inside DNA Genotek
Legal