

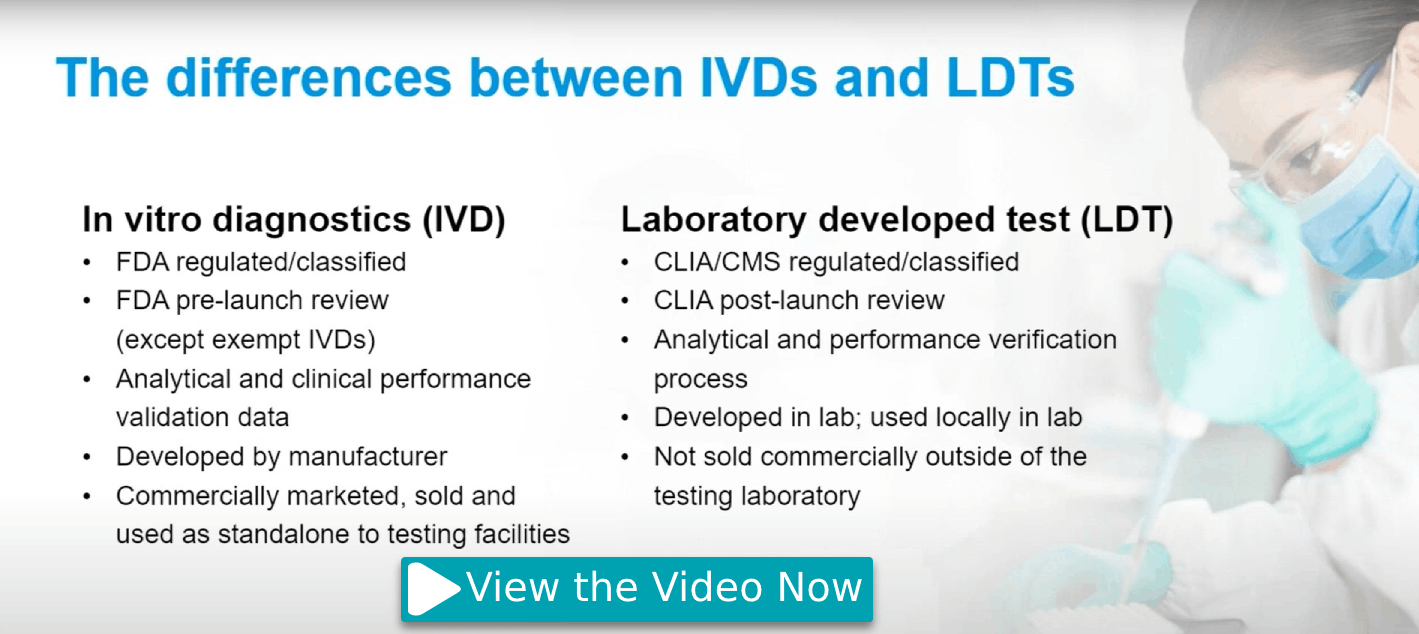
Learn about the value that U.S. FDA-cleared OrageneTM●Dx and ORAcollectTM●Dx saliva collection devices can add to genetic tests and molecular diagnostics, and gain insights into the regulatory landscape for IVDs and LDTs and the strategic path for bringing genetic tests to market while complying with evolving regulations.
Product with 510(k) clearance (e.g., the OrageneTM●Dx and the ORAcollectTM●Dx devices) reduce the time needed to implement devices/assays in prescription or direct to consumer models.
Considerable time and money spent revalidating devices that are not FDA cleared for general use.
*The Oragene™•Dx device and ORAcollect™•Dx device direct-to-consumer (DTC) user comprehension studies (K192920 and K212745, respectively) were conducted to support a pre-market submission to the FDA. These studies were conducted to assess the acceptability of saliva samples collected with the Oragene™•Dx or ORAcollect™•Dx devices for a direct-to-consumer application. The objective of the studies was to demonstrate that naive participants comprehend the instructions for use provided with the devices and can successfully provide a saliva sample at home with acceptable quality for use in downstream diagnostic testing applications.
Some DNA Genotek products may not be available in all geographic regions. Check product availability in your region by selecting your location on the website. Full terms and conditions for all DNA Genotek products are available here. DNA Genotek's sample collection devices and nucleic acid stabilization chemistries are protected by issued and pending patents in numerous countries around the world.
© 2024 DNA Genotek Inc., a subsidiary of OraSure Technologies, Inc., all rights reserved.